|
Chapter 6 Pharmaceutical Affairs |
|
As part of the tradition of oriental medicine, poor separation of prescription and dispensing of drugs have long characterized Japan's medical care, in which doctors directly dispense the drugs to the patients. This tradition has been considerably modified thanks to the government effort through economic incentives by way of manipulating the uniform fee schedule. Japan's pharmaceutical regulation, on the other hand, has sometimes been smeared by iatrogenic disasters involving pharmaceutical adverse effects (ADR), the recent cases of which involve Sorivudine scandals in 1993 and iatrogenic HIV infection through imported blood products in the 1980s. |
|
| 1. Pharmacies and Separation of Prescription and Dispensing | |
|
Traditionally Japanese doctors commonly dispense drugs directly to patients. This is welcomed by both doctors and patients because doctors can raise profit through markups between whole sale prices and reimbursement prices set by the government and patients can save time for receiving all necessary care at a time. This non-separation prescription and dispensing may explain the high share of pharmaceuticals in the National Health Care Expenditure. The government and pharmacist association have long endeavored to enhance the separation by rewarding higher prescription fee to doctors who choose issuing prescriptions rather than directly dispensing since middle of 1970s. However initial efforts to enhance separation between prescription and dispensing were hampered by physicians' conflicts of interest. Many doctors and hospitals established their affiliated pharmacies in their neighborhood and pocketed both higher prescription fee and pharmaceutical markups. To encourage "pure" independence of pharmacies, "carrots and sticks" measures have been taken: penalizing doctors who develop collusive arrangement with pharmacies and rewarding pharmacies that have fewer ties with particular hospitals or doctors. From the health insurance policy, the official reimbursement price has been repeatedly reduced to discourage doctors from direct dispensing by reducing the pharmaceutical markups. Also, generic substitution has been promoted by way of listing the drug with generic names rather than brand names in the official reimbursement list for the products whose patents had expired. For inpatient care, all inclusive per diem reimbursement system is increasingly suppressing the share of pharmaceutical cost in the inpatient health care cost. As part of cost control efforts, prescription only drugs are increasingly switched to OTC, the recent example of which includes H2 blocker. |
|
| 2. Pharmaceutical Prices | |
|
The government sets prices of all drugs reimbursed by the health insurance system. The list of reimbursable drugs includes nearly 14,000 items for both oral, IV and ointment. To calculate the price, the government is authorized by the Pharmaceutical Affairs Act to conduct a market survey every year. This survey is conducted with close cooperation with wholesalers who submit their transaction records with health care providers. The official reimbursement price will set at the weighted average of the transaction price plus the reasonable margin that is usually set at 2%. For newly approved drugs, the price will be set by comparison with other drugs of the same therapeutic effect. For a limited number of innovative drugs, some bonus prices may be awarded on a case-by-case basis. |
|
| 3. Clinical Trials | |
|
Pharmaceutical products, cosmetics and medical equipment are subject to regulation by the Pharmaceutical Affairs Act. The Act was amended in April 1993 to allow public subsidies for research and development of orphan drugs as well as accelerated review. New drug applications will be subject to preliminary review by a special agency named "Pharmaceuticals and Medical Equipment Evaluation Center" and then final review by the Pharmaceutical Affairs Committee. Final decision is left to the discretion by the Minister of Health, Labor and Welfare. Regulations on clinical trials were tightened by the amendment of the Pharmaceutical Affairs Act in June 1996 in response to a series of misconducts exposed in the preceding years. |
|
| 4. Japan's Pharmaceutical Industry | |
The output of Japan's pharmaceutical industry is 6.15 trillion yen or approximately 50 billion dollars in 1997, of which only 47 billion yen or approximately 383 million dollars was exported. On the other hand, Japan imports as much as 621 billion yen or approximately 5 billion dollars from abroad, of which 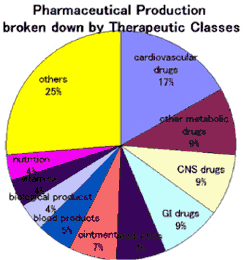 Germany, the U.S., the U.K., Sweden and Denmark constituted roughly 70% in 1997. Broken down by therapeutic class, cardiovascular drugs were produced in the ex-factory value of one trillion yen or approximately 16.9% of the total Japan's pharmaceutical output according to the Pharmaceutical Industrial Statistics. |
|
| 5. Pharmaceutical Monitoring and Surveillance | |
|
Pharmaceutical monitoring and surveillance consists of voluntary reporting from health care providers and compulsory reporting from pharmaceutical manufacturers. The procedure for voluntary reporting was eased in 1997 increasing the number of reporting from previous 1,914 in 1996 to 3,730 in 1997. All reported cases are evaluated by a subcommittee of the Central Pharmaceutical Affairs Committee. MHLW periodically publishes "Pharmaceutical Safety Information" every other month and issues "Emergency Safety Information" in an ad hoc manner. |
|
| 6. Blood Products | |
|
Japan's per capita consumption of blood products are higher than most developed countries. Basically all blood products consumed domestically should be supplied by donated blood. However the donated blood alone is not sufficient to fulfill the domestic demand and majority of the blood necessary for production of plasma fraction products such as albumin and globulin is imported. As of 1998, only 26.4% of albumin and 61.4% of globulin are supplied by domestic blood donation. Japan has seen a sharp increase of blood products consumption in the late 1970s, boosted by huge pharmaceutical markups. This excessive use of blood products may be partially to blame for the spread of the iatrogenic HIV infection in the middle of 1980s. Controlling of excessive use of blood products has been attempted through practice guidelines and utilization reviews. |
|
  
|